
PDRN therapy—short for polydeoxyribonucleotide—has gained popularity for skin rejuvenation and wound support.
In some countries, it is marketed as a “skin booster” and promoted for improving skin texture, elasticity, and recovery.
In Japan, however, patients often ask the same vital question: Is PDRN therapy safe and approved in Japan?
This guide clarifies the current regulatory position, summarizes available safety data, and outlines practical steps to help patients make informed decisions.
What Is PDRN Therapy?
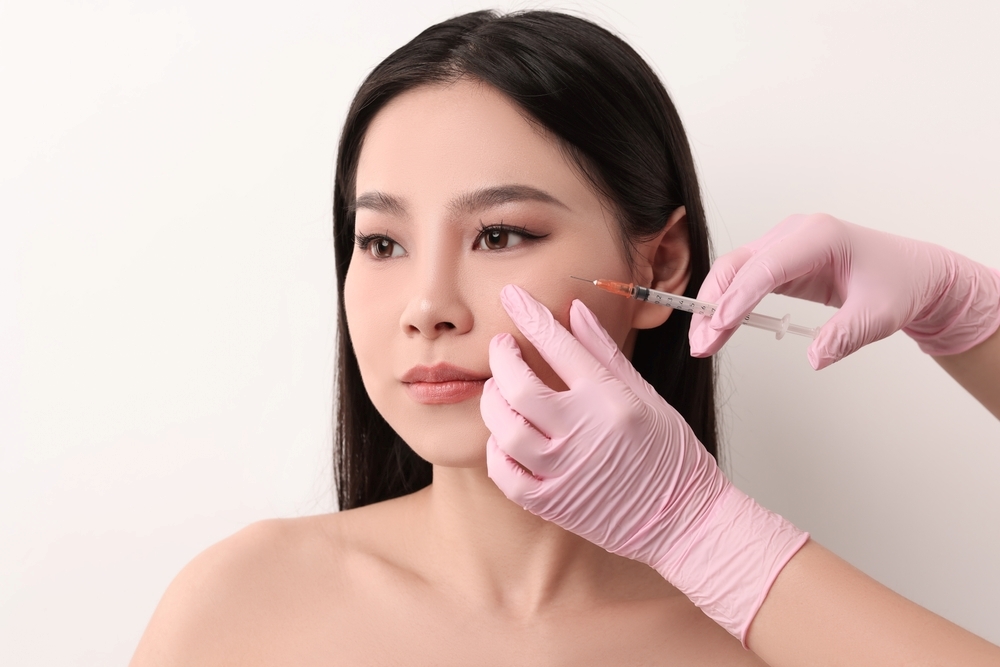
PDRN is a mixture of highly purified DNA fragments, typically sourced from salmon.
In dermatology, it is administered via small intradermal injections intended to activate adenosine A2A receptors, reduce inflammation, and stimulate fibroblasts to produce collagen and extracellular matrix components.
Clinical literature has associated PDRN use with improved tissue repair, skin hydration, and overall skin quality when delivered as a series of treatments, although individual outcomes vary.
Is PDRN Therapy Safe and Approved in Japan?

Regulatory Status with the PMDA
According to publicly available regulatory information, injectable PDRN products used for aesthetic skin rejuvenation do not currently hold marketing approval from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA).
While topical cosmetics referencing salmon DNA may be available domestically, these are distinct from injectable pharmaceutical products and do not indicate approval for injectable use.
As a result, clinics offering PDRN injections typically provide them as private, non-insured treatments using imported products and should not present them as government-approved therapies.
How Clinics Legally Provide PDRN
Under Japan’s private-pay medical framework, medical institutions may use certain unapproved therapies provided they comply with applicable importation, quality control, and disclosure requirements.
Ethical practice requires transparent informed consent that clearly states the product is not approved domestically, outlines known benefits and potential risks, and explains alternative treatments that do carry PMDA approval.
Safety Profile: What the Evidence Shows
Peer-reviewed studies in wound healing and dermatology suggest that PDRN is generally well tolerated when manufactured under strict sterility standards and administered by trained clinicians.
Common, transient reactions include redness, pinpoint bleeding, swelling, tenderness, and bruising at injection sites.
Less common risks include infection, nodule formation, delayed inflammatory reactions, or pigmentation changes.
Because PDRN is derived from fish DNA, individuals with severe fish or seafood allergies should discuss suitability with a physician.
As with any injectable treatment, clinic hygiene, aseptic technique, and emergency preparedness are critical determinants of safety.
Who Should Exercise Caution
Patients who are pregnant or breastfeeding, those with active skin infections, uncontrolled autoimmune conditions, bleeding disorders, or those taking anticoagulant medications should seek individualized medical advice.
A thorough medical history review, allergy assessment, and expectation-setting consultation are essential before proceeding.
Quality, Sourcing, and Transparency
Clinical outcomes depend heavily on product quality and injection technique.
Reputable clinics document the product’s official name, lot numbers, country of manufacture, sterility validation, and storage conditions.
They also provide written explanations of indications, treatment intervals, and aftercare.
Patients should be cautious of vague product descriptions or exaggerated claims of regulatory approval.
What to Ask Your Clinic Before You Book
- What is the exact product name, country of origin, and PMDA regulatory status?
- Who performs the injections, and what training and experience do they have with PDRN?
- How many sessions are recommended, at what intervals, and what results are realistic?
- What are the common side effects, rarer risks, and emergency response protocols?
- How are sterility, traceability, and cold-chain storage maintained?
- What PMDA-approved alternatives could address similar concerns?
Alternatives in Japan
Patients who prefer treatments with domestic approval may consider PMDA-approved hyaluronic acid fillers for hydration and fine-line improvement, as well as energy-based devices such as lasers, radiofrequency (RF), or IPL systems with recognized clearances for improving texture, tone, and collagen remodeling.
Autologous procedures such as platelet-rich plasma (PRP) may also be discussed, noting their distinct regulatory frameworks and evidence base.
Conclusion
So, is PDRN therapy safe and approved in Japan?
When administered by experienced clinicians using high-quality products, PDRN appears generally well tolerated.
However, injectable PDRN for aesthetic purposes is not broadly PMDA-approved in Japan and is typically offered under private-pay arrangements with informed consent.
Patients considering PDRN should prioritize provider transparency, product provenance, and a clear discussion of risks and benefits.
Those who prefer only domestically approved options can explore established fillers or device-based therapies, while patients open to unapproved imports should proceed carefully and select providers with strong safety standards.
| ・This website provides general knowledge about aesthetic medicine from a neutral perspective as much as possible. Please note that the information is not intended to encourage self-diagnosis. Be sure to check the official website of the clinic and consult each medical institution for details regarding treatment. ・This article is based on information available at the time of writing and publication. Please check the official website for the latest updates. ・If cosmetics or massage-related content is mentioned, it is not within the scope of medical supervision. |







